Tackling the global burden of mental health through data-driven research
Depression is the leading cause of disability and existing therapies have limited efficacy. Stress is a major risk factor for depression although not all those who experience stress become depressed. The field of neuroscience is witnessing unprecedented technological advances, offering previously unimaginable power to probe brain function. In contrast, the understanding of mechanisms of depression has advanced little. Research in the Bagot Lab for Behavioural Neurogenomics employs an integrative circuit-dissection approach combining molecular, cellular and behavioral technologies to determine how experience-dependent plasticity in stress-sensitive neural circuits shapes emotional, motivational and cognitive alterations observed in depression. Unravelling the dynamic interplay between brain circuits and gene networks in behavioural control will enable development of novel circuit-based treatments to treat, or even prevent, psychopathology in susceptible individuals.
Techniques in the lab include
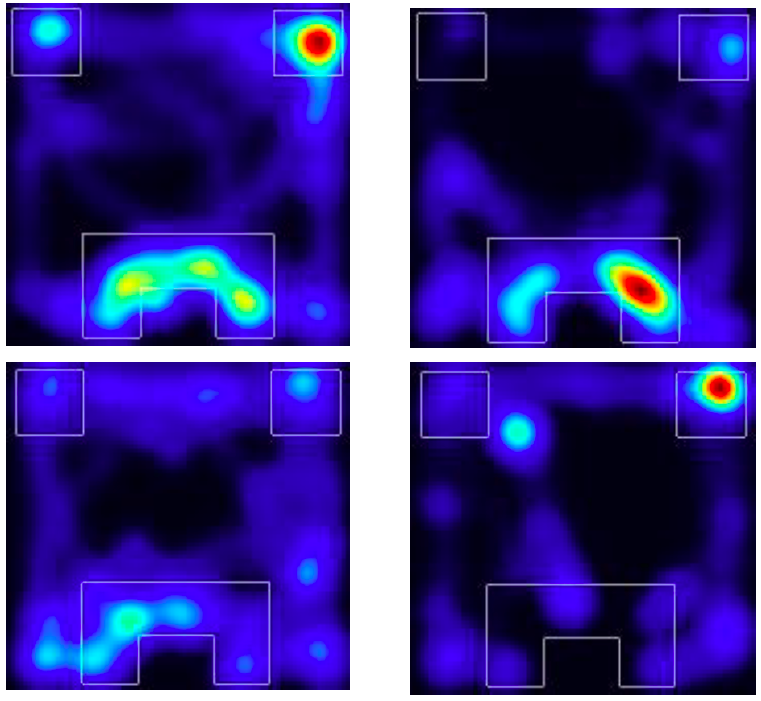
Mouse operant behaviour
Standard tests of anxiety- & depression-like behaviour
Computational behavioural analysis
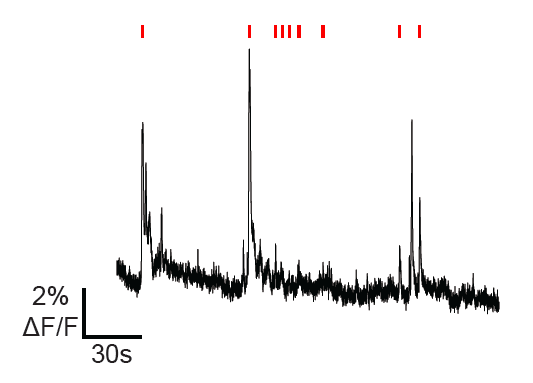
In-vivo calcium imaging
Circuit- and cell-type specific optogenetic & chemogenetic manipulation
Ex vivo electrophysiology
Viral-mediated gene manipulation
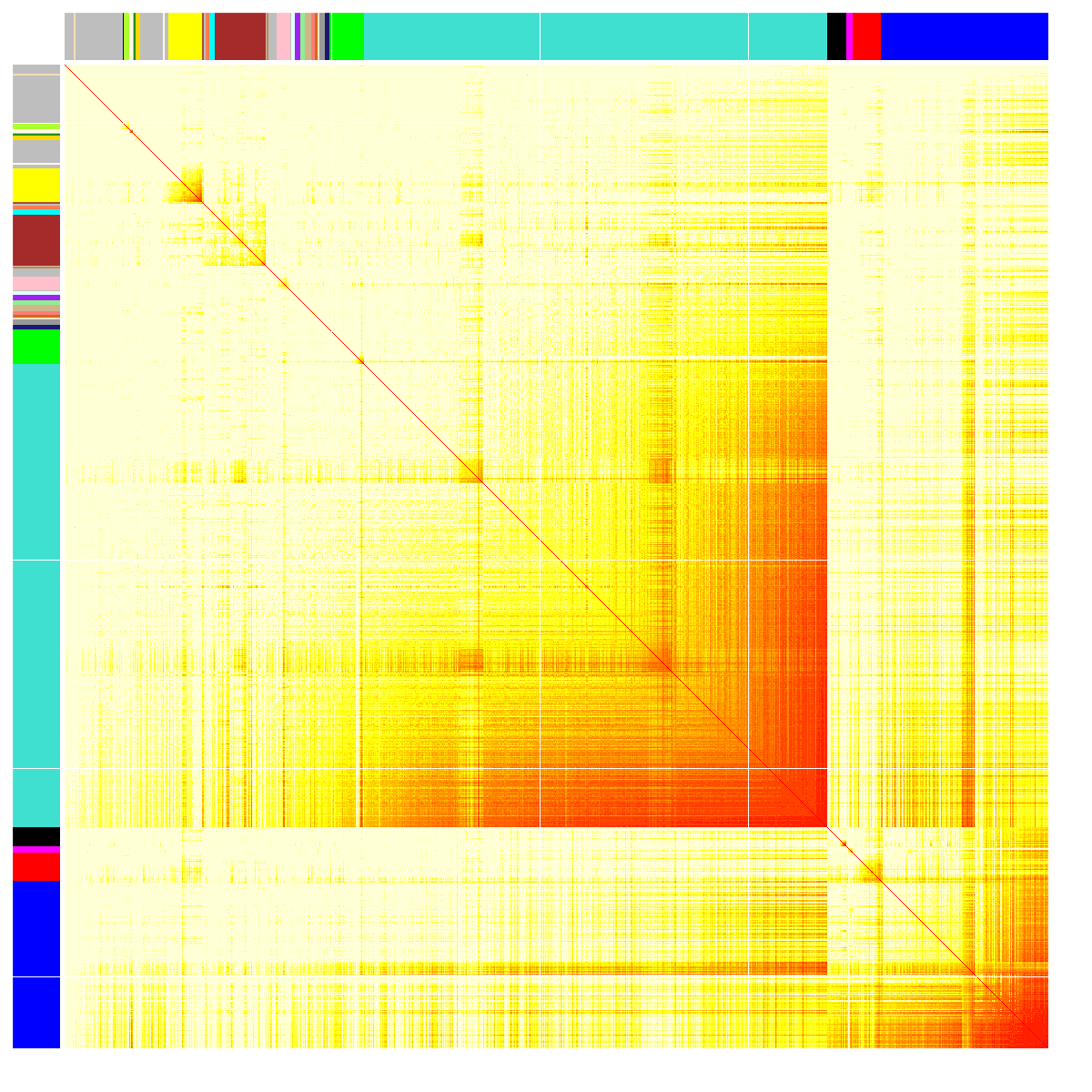
Next-generation sequencing and bioinformatics analysis
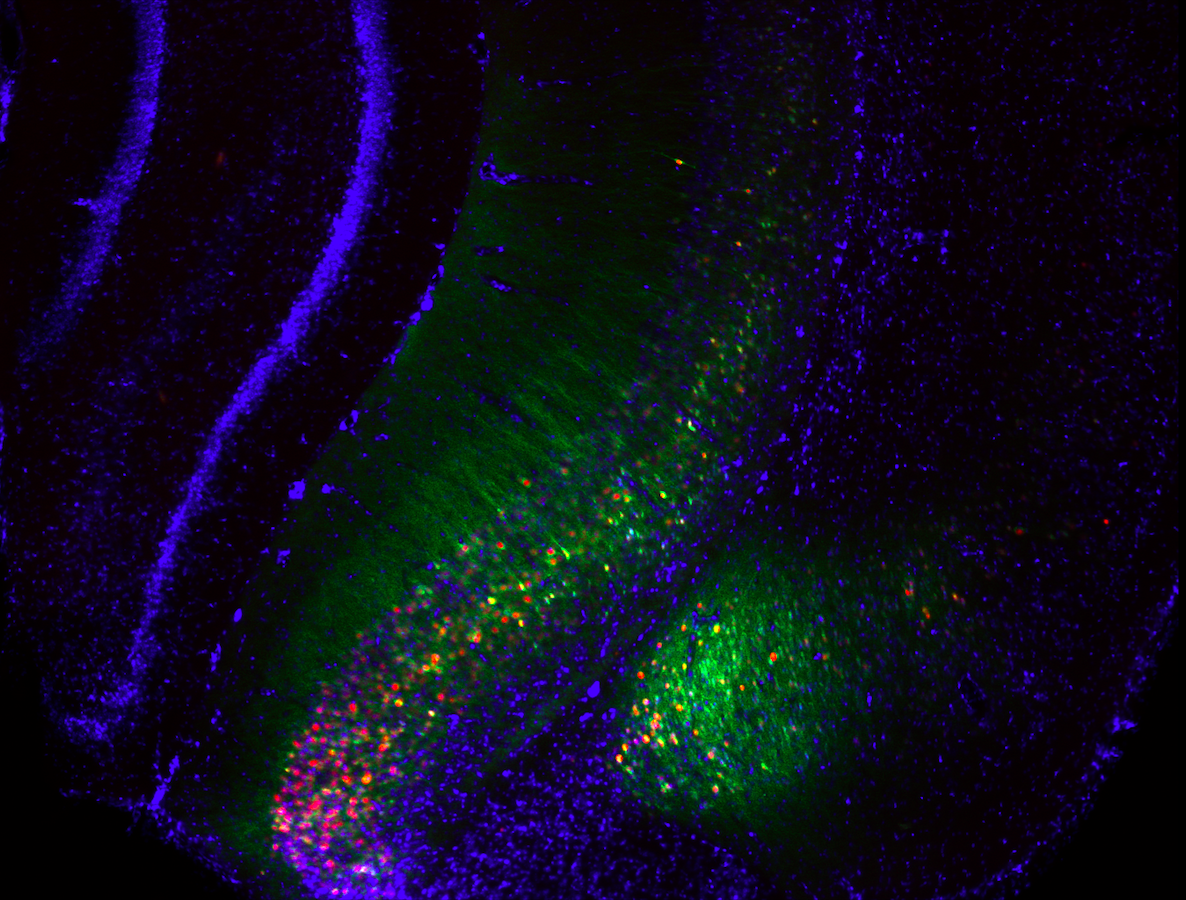
RNAscope, qRT-PCR, immunohistochemistry
Current research directions in the lab include
IDENTIFYING DIFFERENTIAL VULNERABILITY TO STRESS
Depression is a chronic, debilitating condition and increasing chronicity associates with worsening symptom severity, increased neural changes, and poorer prognosis for treatment. While life stress is a major risk factor, not all people who experience intense life stress develop depression. Predicting the individuals most at risk of developing psychopathology before the full syndrome emerges, opens a window of opportunity for early interventions, that may be more effective than traditional treatment approaches treating observable symptoms of the disorder. To uncover the mechanistic basis of vulnerability, we are interrogating neural signatures of future susceptibility before stress and before overt depression-like behaviour emerges. We are developing innovative behavioural paradigms to model behavioural endophenotypes observed in human depression to study the neural circuit mechanisms that predispose certain individuals to depression.
SEX, STRESS & SUSCEPTIBILITY
The incidence of depression and anxiety is significantly elevated in women, and stress is a major risk factor. Mirroring the increased incidence of stress-related disorders in women, female rodents are more susceptible to chronic stress yet until recently the vast majority of basic neuroscience has focused exclusively on males. We are developing models of brain function integrating high-resolution analysis of gene activity with unbiased analyses of behaviour to determine how chronic stress increases susceptibility to stress-related psychopathology, in both males and females.
STRESS, LEARNING & DECISION MAKING
Adaptive behaviour requires optimising reward and limiting aversive outcomes across changing environmental conditions. Individual differences in learning about rewarding and aversive outcomes and how this learning influences decision making are associated with depression, anxiety and other psychopathologies. Using robust mouse behaviour paradigms, we are probing the neural circuit mechanisms by which stress interacts with learning and decision making.
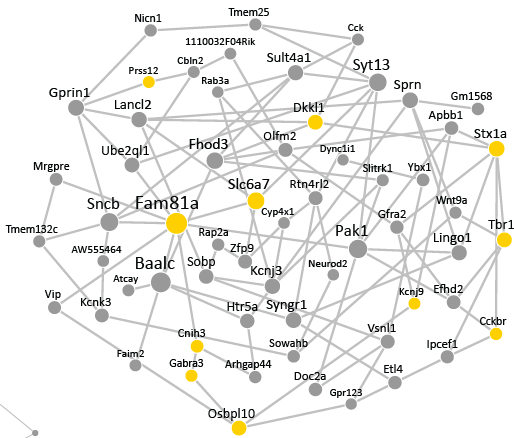
TRANSCRIPTIONAL MEDIATORS OF STRESS-INDUCED CIRCUIT CHANGE
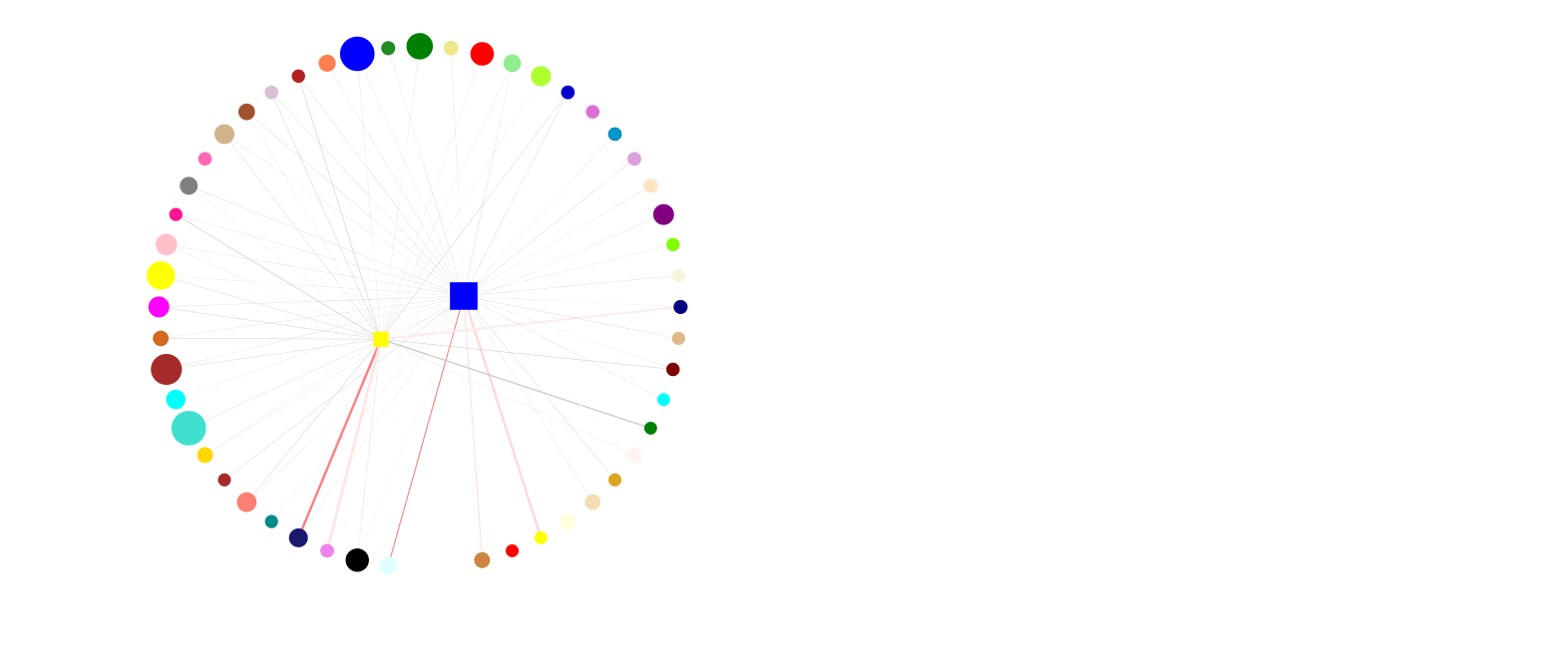
We must continually adapt our behaviour to respond to changing environmental conditions. Experience-induced changes in gene expression are key to understanding the molecular mechanisms by which experience guides behavioural adaptation. Accumulating data demonstrates that complex behaviours are mediated by activity in functionally interconnected brain circuits, and not just single brain regions. Increases or decreases in gene expression regulate neural activity to alter behaviour yet relatively little is known about how gene expression changes regulate brain circuits during behaviour or how groups of genes interact to influence neuronal activity. We are examining how networks of co-expressed genes control neuronal activity within brain circuits important for control of emotional behaviour, learning and stress susceptibility.